Role of Oxygenation in Barrel Aged Spirits – Part II
In Oxygenation Part I, we discussed different variables that influence the rate of oxygenation during the maturation period. In this post, we switch gears and focus on what kind of reactions we can expect to see as a product of the oxygenation process.
If we analyzed even just a few milliliters of a barrel aged spirit, we would discover a vast array of chemical compounds. Some of these compounds produce specific flavors or aromatics, others contribute very little, and for a significant portion of the compounds, we still don’t completely understand the impact they have on the spirit. The extent to which oxygen interacts with these different compounds is largely unknown but widely speculated.
When speaking with Dr. Seth DeBolt, director of the distillation, wine and brewing program at the University of Kentucky, he reinforced this by saying:
“Components such as fatty acids and sugar aldehydes can interact with oxygen to form carboxylic acids but the degree to which this is taking place and perception in the overall flavor profile is difficult to judge.”
In other words, we know these reactions are occurring throughout the maturation process, but the frequency and organoleptic perception is hard to discern.
Oxygenation is largely attributed to the fruity and floral notes found in spirits and this is true in some cases. Most of the aromas found in spirits that one might characterize as fruity or floral are formed by a process called esterification. Esters are chemical compounds (see figure 1) normally formed from the combination of an alcohol and an acid.
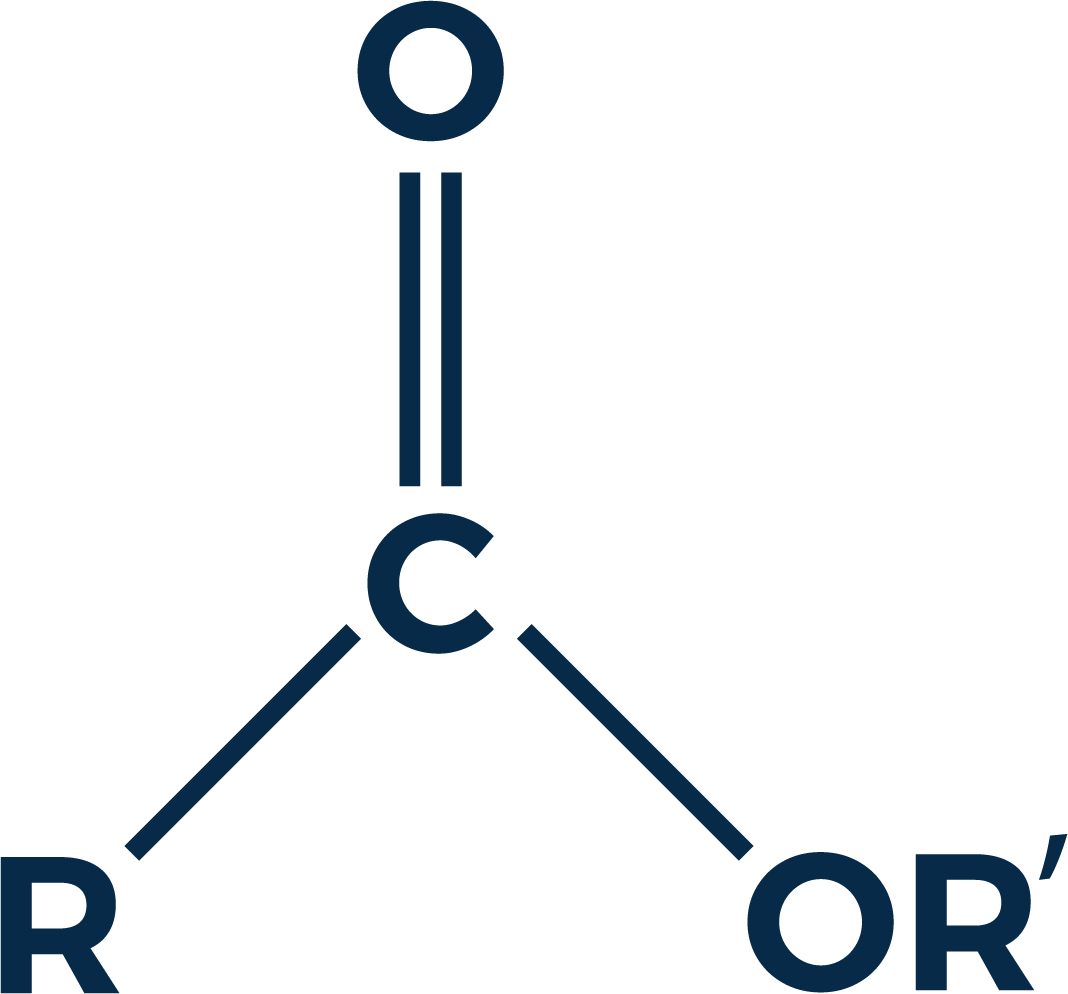
Esters occur naturally and are also manufactured on an industrial scale to produce common products such as solvents, oils, fragrances, softening agents and mylar¹. The same esters found in nature can also be found in whiskey. Most esters have pleasing aromas even though some of the acids from which they are born are very unpleasant. For example, ethyl butyrate has a fruity odor similar to pineapple, however one of its components, butyric acid, smells like vomit.
Ethyl acetate is the most common ester found in barrel aged spirits although it is not the most aromatic. Its formation is outlined in figure 2. Ethyl acetate is a solvent commonly used in nail polish remover and has a pleasant fruity aroma.
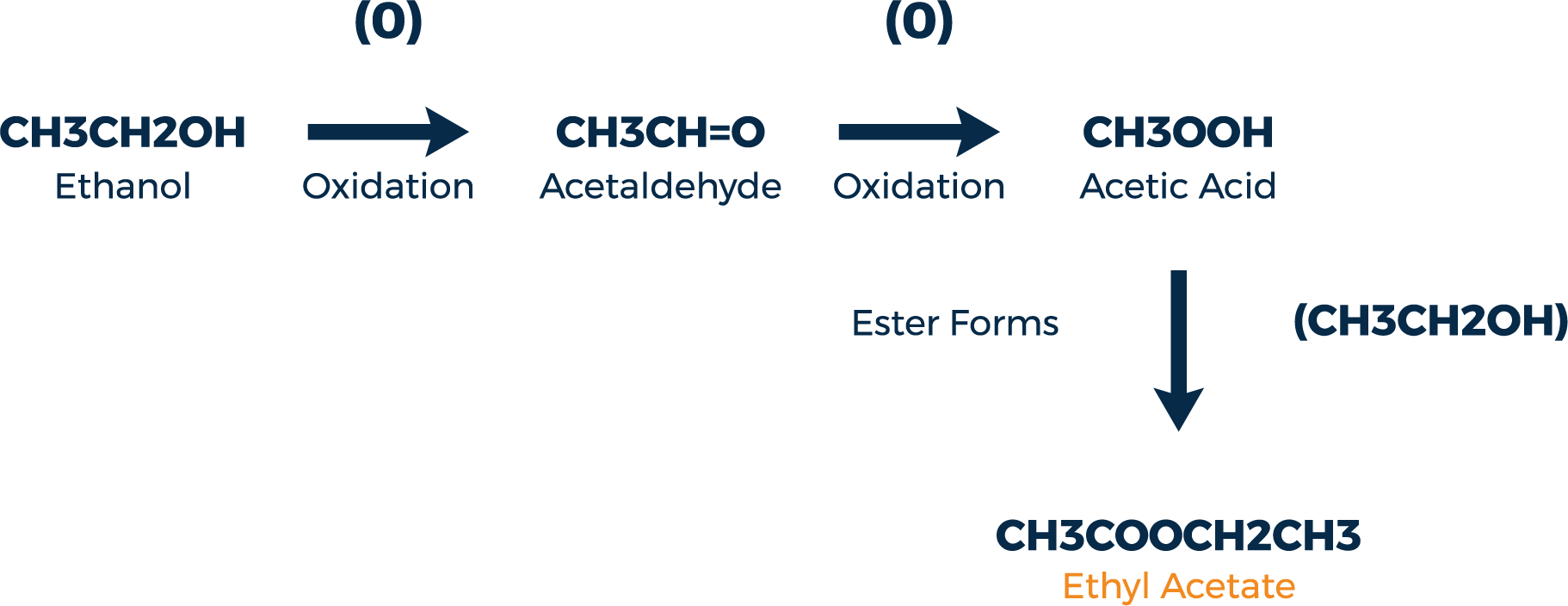
From this example we can see how oxygen plays an indirect role in the formation of esters. While not directly responsible for the ester formation itself, it does help provide the necessary acids that must be present. An important point to make is that ester formation is a constantly evolving occurrence within the barrel. Through a process called transesterification, newly formed esters can combine with an alcohol and form a different ester.

Oxygen can also interact with cask extractives to form new flavor compounds. Coniferaldehyde, a compound that produces a somewhat sweeter wood aroma can be oxidized to form vanillin. The newly formed vanillin can be oxidized into vanillic acid³ and with vanillic acid now present, there is an opportunity for new esters to form through combination with an alcohol.
Oxygen, in part, is also responsible for some of the color derived from the maturation process. To what extent, I am not positive, but we do know there are acids formed by oxidative reactions that will lend some degree of color to the liquid in the barrel. One compound, syringaldehyde, which comes from lignin degradation, will not only lend some sweet flavors to the spirit, but when oxidized, forms syringic acid. This compound will lend an earthy note as well add some color to the spirit.
As stated in the previous post, oxygenation is a process that we have some control over but as we can see from the interactions above, this control is limited. We can push the cart to the top of the hill so to speak, but after that it is on its own.
Cheers,
Andrew
- The Editors of Encyclopedia Britannica. “Mylar.” Encyclopedia Britannica, Encyclopedia Britannica, Inc. 18 May 2016 www.britannica.com/topic/mylar.
- G Reazin (1981) Chemical mechanisms of whiskey maturation. American Journal of Enology and Viticulture, 32 (4), 283-9.
- Nishimura, M Ohnishi, M Masuda, K Koga, R Matsuyama (1983) Reactions of wood components during maturation. Flavor of Distilled Beverages: Origin and Development, Ellis Horwood Ltd. Chichester,pp.225-240.


